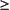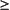CML
Response defined
The following categories define treatment response in CML :
•Complete hematologic response: White blood cell count <10,000/microL with no immature granulocytes and <5 percent basophils on differential, platelet count <450,000/microL, and spleen not palpable.
•Complete cytogenetic response: No Philadelphia chromosome positive cells present.
•Partial cytogenetic response: 1 to 35 percent Philadelphia chromosome positive cells present.
•Major cytogenetic response includes patients with complete and partial cytogenetic response (ie, 0 to 35 percent Philadelphia chromosome positive cells present).
•Minor-minimal cytogenetic response: 36 to 95 percent Philadelphia chromosome positive cells present.
•Complete molecular response: BCR-ABL transcript nondetectable and nonquantifiable in an assay that has at least 4 to 5 log range of detection.
•Major molecular response: An at least three log reduction in BCR-ABL transcript levels from a standardized baseline (which varies by laboratory).
Treatment failure is defined by
the inability to reach the following threshold levels after the initiation of therapy:
•Any hematologic response by three months
•Complete hematologic response by six months
•Partial cytogenetic response by twelve months
•Complete cytogenetic response by eighteen months.
A suboptimal response occurs when there is less than a:
•Complete hematologic response by three months
•Minor cytogenetic response by six months
•Complete cytogenetic response by twelve months
•Major molecular response by eighteen months
Monitoring schedules
One approach to monitoring disease in patients in chronic phase is the following:
•After a patient is diagnosed with chronic phase CML, monitor weekly complete blood counts with differential until these counts are stable.
•If the patient achieves a complete hematologic response and the counts are stable, perform complete blood counts, chemistries, and quantitative RT-PCR or FISH on the peripheral blood every three months. Bone marrow cytogenetics are performed when the peripheral blood FISH is zero to five percent positive or the RT-PCR is at a value corresponding to a complete cytogenetic response.
•If the patient achieves a complete cytogenetic response, the following is performed every three to four months: complete blood count with differential and quantitative RT-PCR or FISH on the peripheral blood. Bone marrow cytogenetics should be repeated when there are significant rises in the peripheral blood quantitative PCR. Some centers advocate bone marrow cytogenetics every 18 to 24 months for patients in complete cytogenetic response to evaluate for the presence of clonal abnormalities in Philadelphia chromosome negative cells. Since there is no consensus on the clinical impact of such changes, this area remains controversial.
Evaluation at loss of response
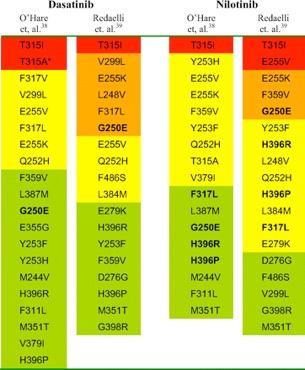
When there is evidence for a loss of response to therapy, the disease phase should be re-evaluated with a bone marrow biopsy with cytogenetics. In addition, patients with either an inadequate response to imatinib or in whom there is a loss of response, mutational analysis of BCR-ABL is recommended. A newly acquired mutation in BCR-ABL may trigger a change in treatment (eg, dose increase, change to another TKI, HCT) depending upon the type of mutation found.
Mutational analysis of BCR-ABL should be performed for all patients who fail imatinib. Although clinical data describing the relative benefits of imatinib or dasatinib in patients with different mutations are just being analyzed, this data may help guide the choice of therapy in the future. Notable examples include:
•The T315I mutation has shown resistance to all currently available TKIs. Such patients should either proceed to transplantation or try an investigational agent as part of a clinical trial.
•The Y253H mutation is resistant to imatinib and nilotinib but sensitive to dasatinib.
-
• The F317L mutation is sensitive to nilotinib but shows intermediate sensitivity to imatinib and dasatinib.