Prognostic features in chronic lymphocytic leukemia
Good prognostic features
1.Low Rai or Binet clinical stage
2.Interstitial or nodular pattern of lymphocyte infiltration in marrow
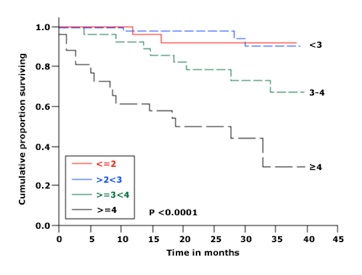
3.Lymphocyte doubling time >12 months
4.CD 38 negativity
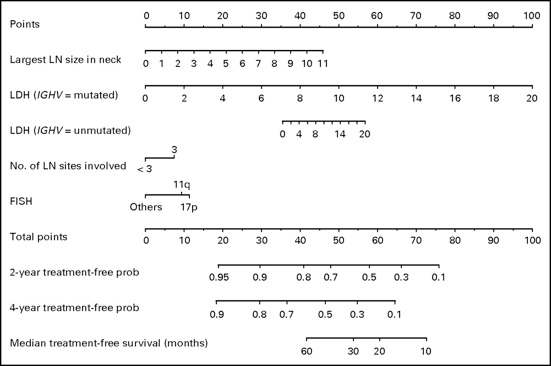
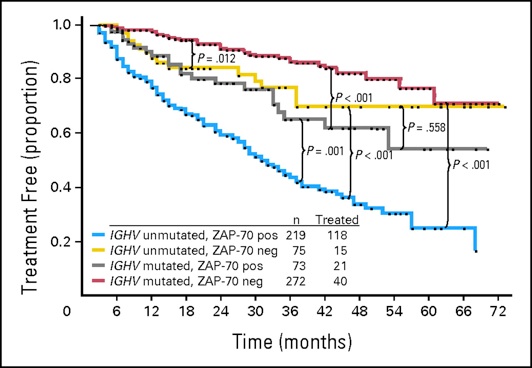
5.Mutated immunoglobulin Vh genes
6.ZAP-70 negativity (low levels)
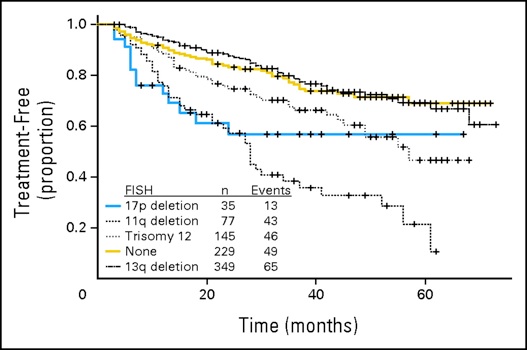
7.Chromosome 13q14
Poor prognostic features
1.High Rai or Binet stages
2.Diffuse pattern of lymphocyte marrow infiltration
3.Lymphocyte doubling time <12 months
4.CD 38 positivity
5.Unmutated immunoglobulin Vh genes
6.ZAP-70 positivity (high levels)
7.Del 11q23
8.17p-/p 53 abnormalities
9.p 53 dysfunction or increased expression
10.Increased levels of TNF-alpha, beta-2 microglobulin, IL-6, IL-8, IL-10, LDH, VEGFR-2, CD20, and CD52